What is known about BRCA mutated breast cancer?
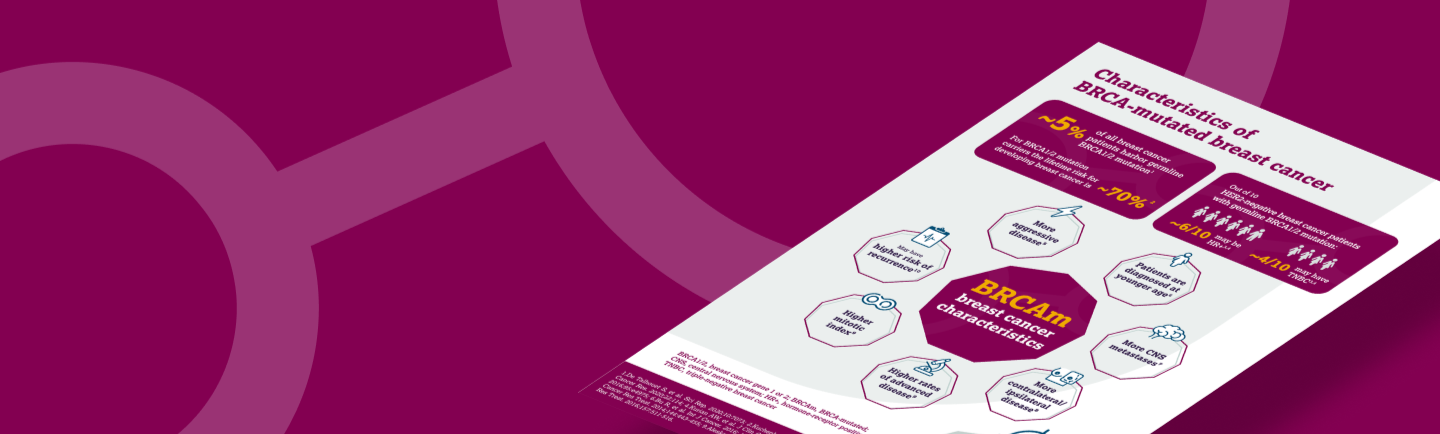
BRCA-mutated breast cancer can be considered high risk due to its characteristics:
- Pathogenic mutations in breast cancer (BRCA) genes are associated with increased risk of cancer.1-3 While lifetime risk for developing breast cancer in the general female population is 5%, mutations in BRCA1 and BRCA2 genes increase the risk to approximately 70% (72% for BRCA1 and 69% for BRCA2).1,2
- Patients with BRCA-mutated (BRCAm) breast cancer also have distinct tumor characteristics compared with the sporadic population:4-8 BRCAm breast cancer is more aggressive, the patients are younger at the diagnosis (BRCAm, 40–45 years;9,10 non-BRCAm, 63 years11), tumor grade is higher with increased Ki67 proliferation marker expression, patients have more CNS metastases and more contralateral or ipsilateral disease compared with the sporadic breast cancer population.4-8
- Patients with BRCA1/2 mutations also have higher rates of advanced disease, higher mitotic index than non-mutation carriers, and are most likely to have a high (BRCA1) or intermediate (BRCA2) risk of recurrence score.6,12