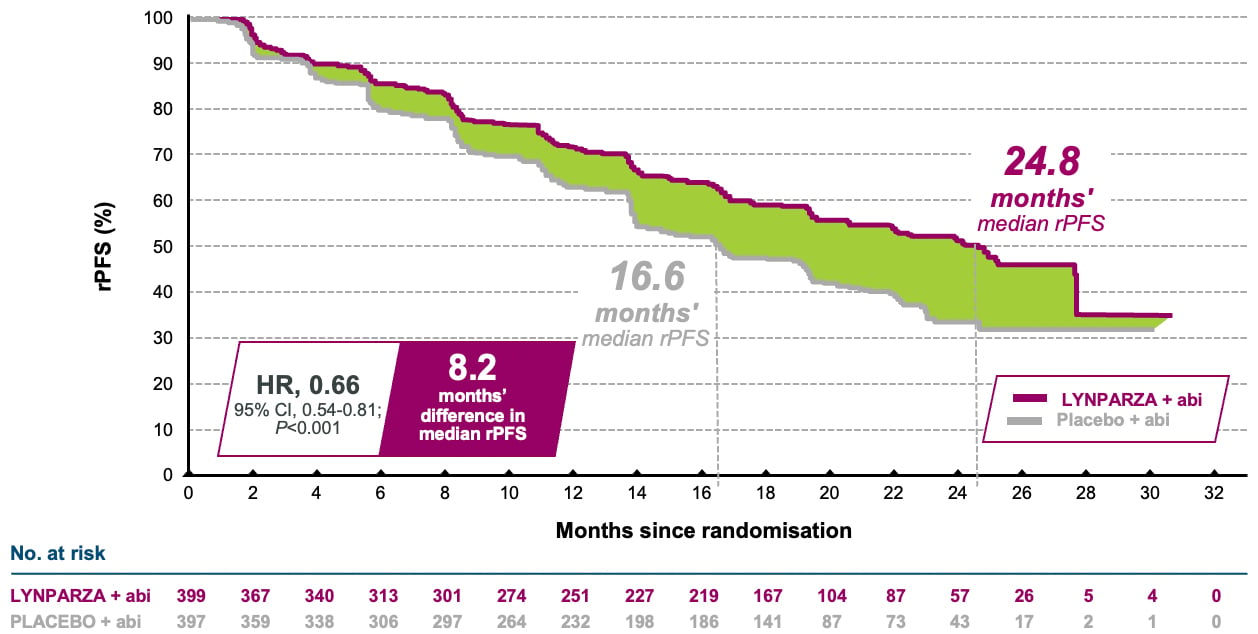
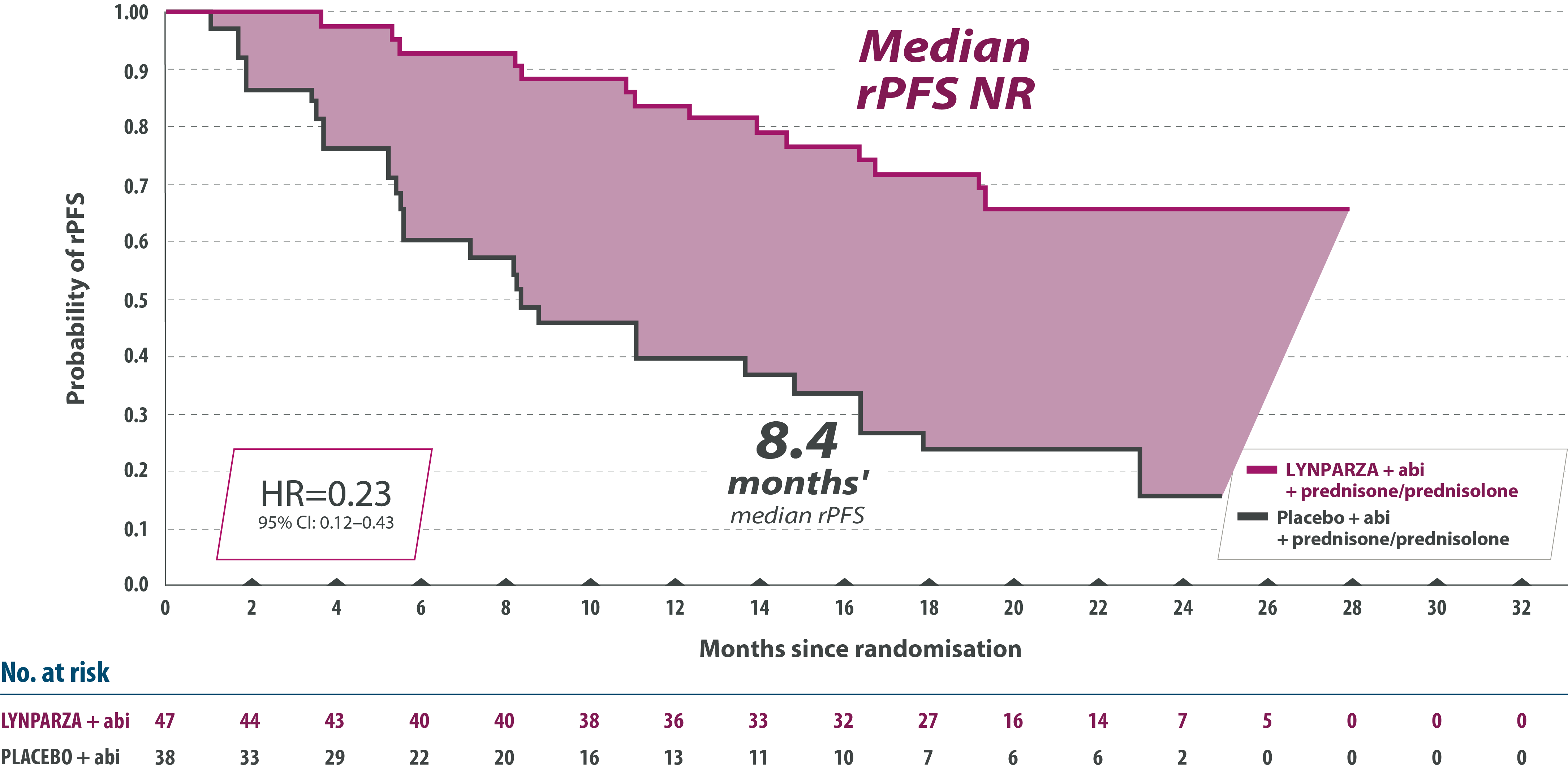
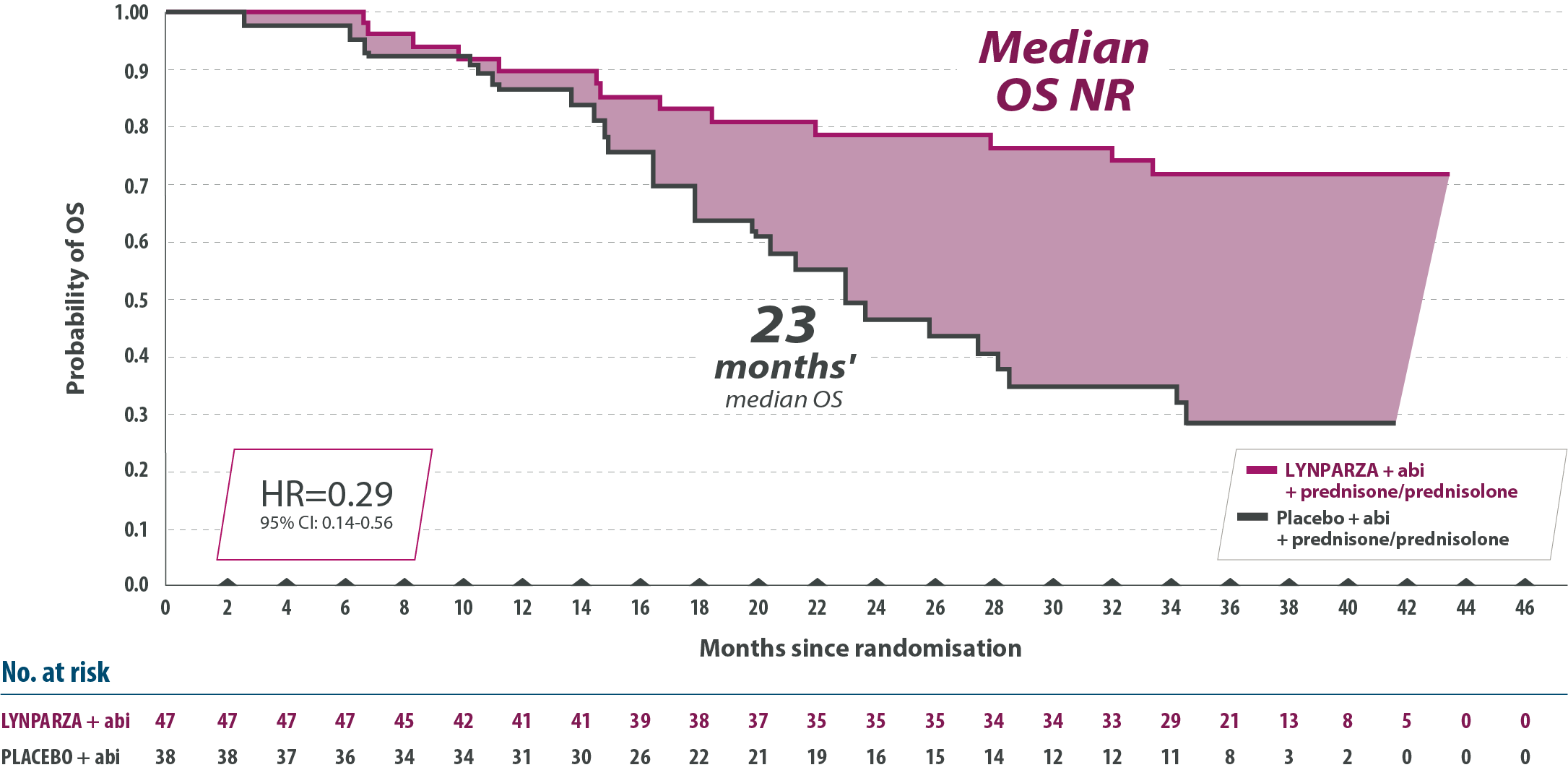
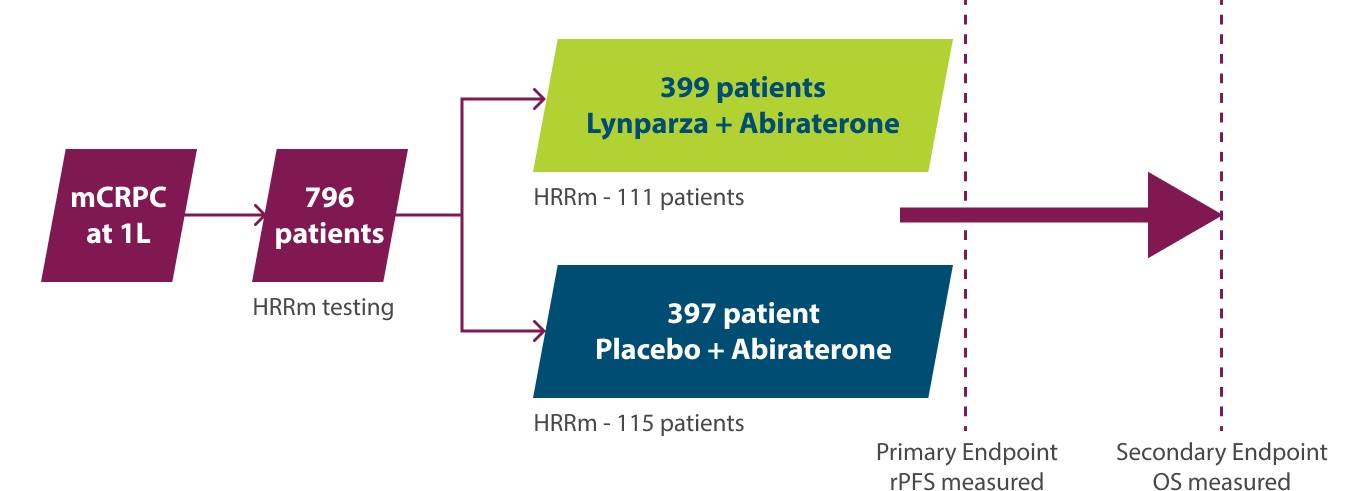
LYNPARZA + abiraterone increased median rPFS by 8.2 additional months versus abiraterone alone1
PROpel is a randomised, double-blind, placebo-controlled, multicentre Phase III clinical trial assessing the safety and efficacy of Lynparza (Olaparib) in combination with abiraterone against placebo-abiraterone as a first-line (1L) treatment alternative for patients with metastatic castration-resistant prostate cancer (mCRPC).2
Previous investigations in phase II clinical trial have highlighted the potency and the synergistic anti-tumor effect of combining Lynparza (olaparib) with next generation hormonal agent abiraterone in improved treatment response in mCRPC patients.3 Lynparza (olaparib) is the first PARP inhibitor approved in the European Union (EU) for treatment of patients with mCRPC with BRCA1/2 mutations, a subpopulation of homologous recombination repair (HRR) gene mutations in November 2020, based on a subgroup analysis of the PROfound Phase III trial.1,4
The PROpel trial
This trial was designed to analyse the clinical response at 1L in mCRPC patients, comparing the combined Lynparza(olaparib)-abiraterone (+prednisone (n=399)) regimen against placebo-abiraterone (+prednisone (n=197)), irrespective of homologous recombination repair (HRR) genes mutational status. Dosage of this combination therapy is the same as to dosage of individual monotherapy. The primary endpoint was measured using radiographic progression-free survival (PFS), assessed by the investigator. Additionally, pre-specified sensitivity analysis of rPFS was performed by blinded independent central review (BICR). Overall survival (OS) was key secondary endpoint. Analysis was performed for entire clinical cohort as well as the divided equally in the two trial arms.2