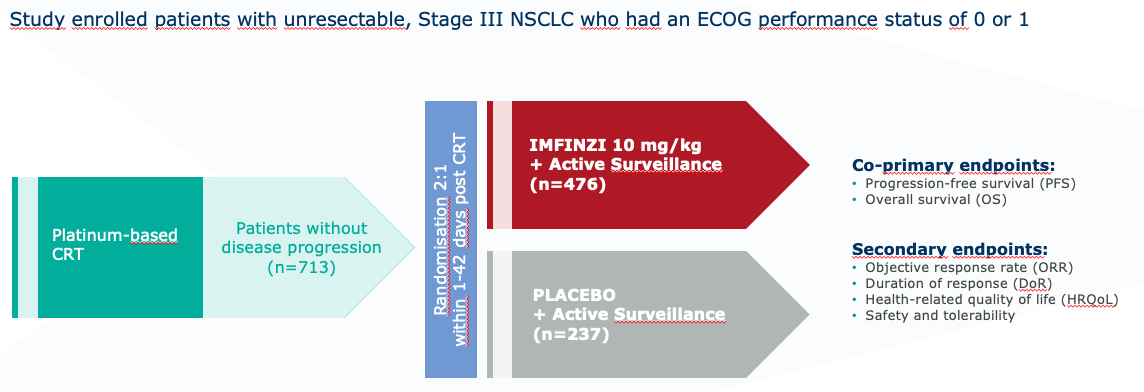
PACIFIC study design1
PACIFIC Updated OS (PD-L1 ≥1% population*)
Imfinzi – Overall survival at 50.1% after 5 years2
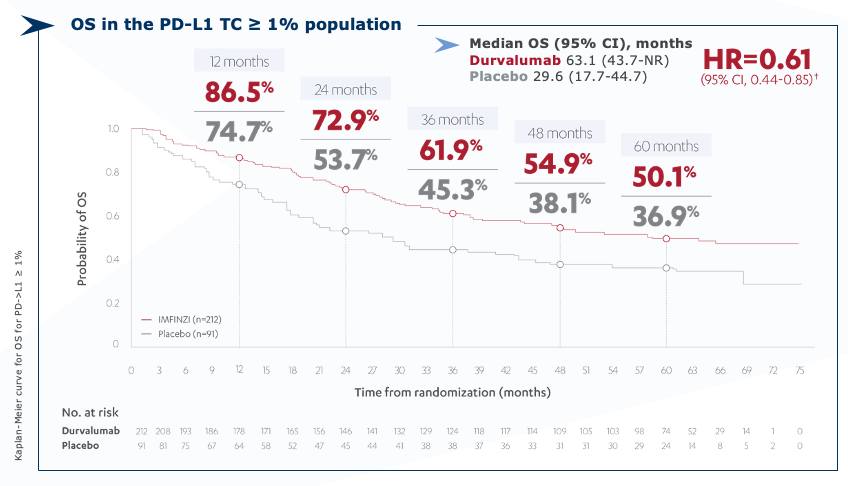
* Exploratory, post-hoc subgroup.
CI=confidence interval; HR=hazard ratio; NR=not reached; OS=overall survival; PD-L1=programmed cell death-ligand 1; TC=tumor cell.
† Treatment effect estimated using an unstratified Cox proportional hazards model.
PACIFIC updated PFS (PD-L1 ≥ 1% population*) (BICR)
Imfinzi – 35.8% of patients have not progressed after 5 years2
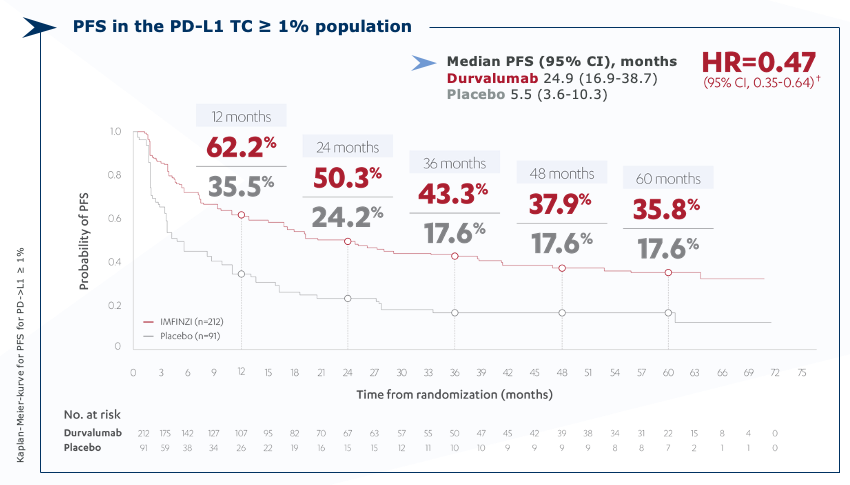
* Exploratory, post-hoc subgroup.
CI=confidence interval; HR=hazard ratio; NR=not reached; PD-L1=programmed cell death-ligand 1; PFS=progression-free survival; TC=tumor cell.
† Treatment effect estimated using an unstratified Cox proportional hazards model.
Exploratory 5 years progression-free survival (PFS) data from PACIFIC study (ITT)
Exploratory 5-year PFS rate was 33% with IMFINZI vs. 19% with placebo3
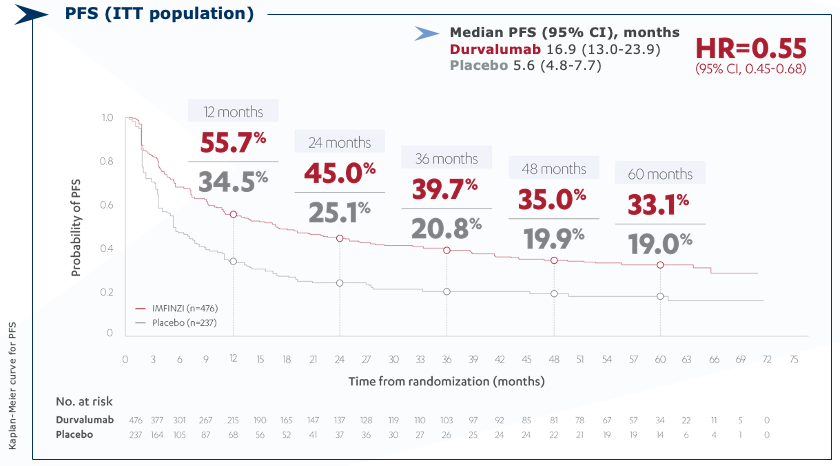
CI=confidence interval; HR=hazard ratio; ITT=intention-to-treat; NR=not reached; PFS=progression-free survival.
PACIFIC study
Progression-free survival (PFS) across prespecified subgroups (ITT population)3
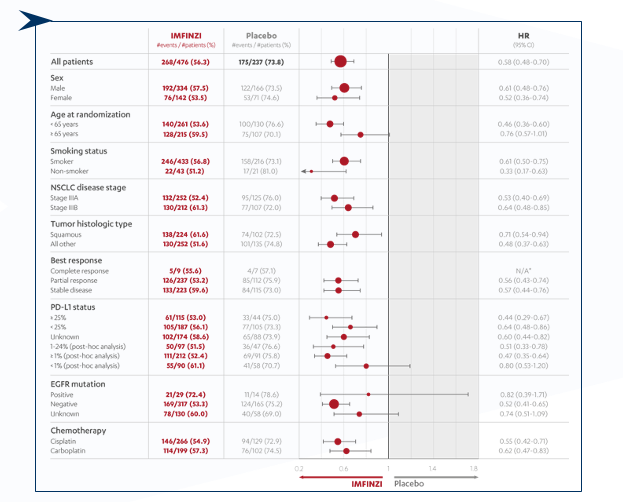
CI=confidence interval; HR=hazard ratio; ITT=intention-to-treat; PFS=progression-free survival.
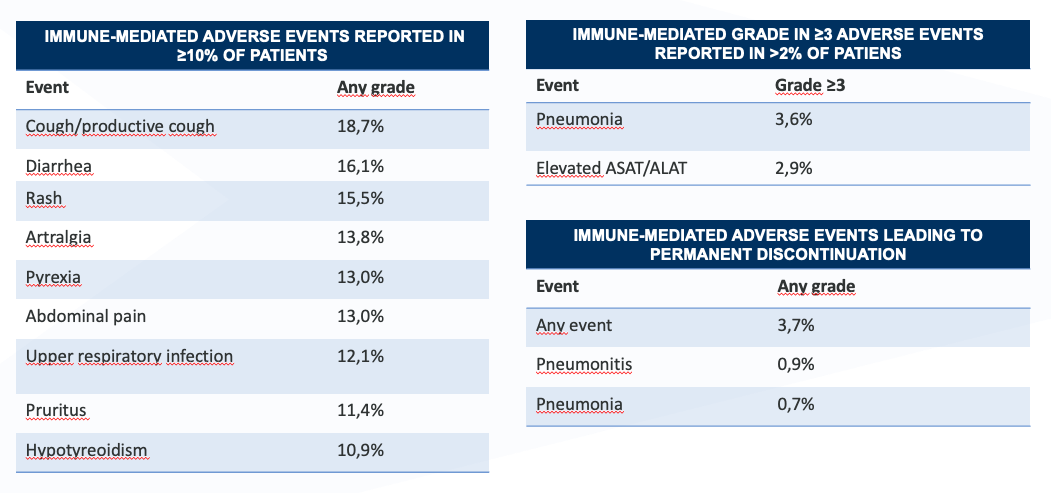
Safety profile of IMFINZI in monotherapy (pooled analysis n=4045)4
Safety profile in PACIFIC - pneumonitis4
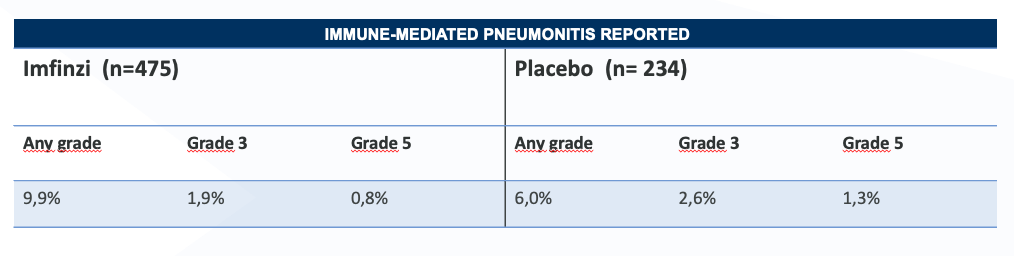
In the PACIFIC study,
IMFINZI vs. placebo following CRT1
Any-grade immune-mediated adverse events reported in ≥1% of patients1
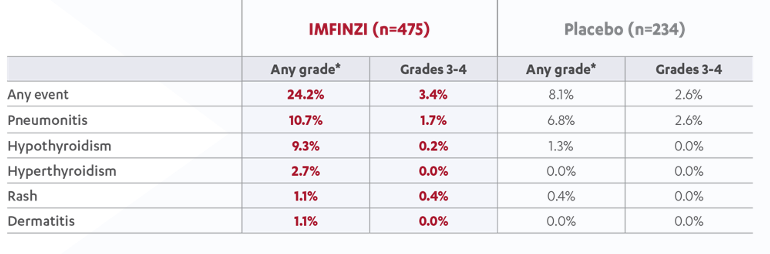
* Grade 5 immune-mediated Aes occured in 4 patients (0.8%) recvieving durvalumab and 3 patients (1.3%) recieving placebo.