IMFINZI (durvalumab) improves OS and PFS vs placebo
THERAPEUTIC INDICATION
IMFINZI as monotherapy is indicated for the treatment of locally advanced, unresectable non‑small cell lung cancer (NSCLC) in adults whose tumours express PD-L1 on ≥ 1% of tumour cells and whose disease has not progressed following platinum‑based chemoradiation therapy.
IMFINZI: THE ONLY IMMUNOTHERAPY APPROVED IN STAGE III NSCLC FOLLOWING CRT1
- Standard of Care for stage III unresectable patients is CRT with curative intent3. Despite that, the 2-year survival is 20%15.
- By adding IMFINZI following CRT, an improved OS and PFS were demonstrated vs placebo in ITT patient1.
- Grade 3 or 4 immune-mediated adverse reactions were 3,4% with IMFINZI vs 2,6% with placebo12.
Approximately 30 percent of the patients with NSCLC present with stage III
Patients with Stage III NSCLC represent a heterogeneous population, and the majority have unresectable tumours.
The IASLC Staging manual in thoracic oncology, Version 8 subdivides stage III NSCLC into IIIA, IIIB, and IIIC presentations5
Imfinzi Mode of Action
In pre-clinical models, synergistic effect when combining immunotherapy and radiotherapy.9
-
-
Radiation induces tumour cell death, releasing a diverse array of tumour antigens10,11
-
-
As a result, PD-L1 is upregulated, inhibiting T cell activity and promoting tumour regrowth8,9
-
-
IMFINZI selectively blocks the interaction of PD-L1 with PD-1 and CD80, which enhances antitumour immune responses1
CD80: cluster of differentiation 80; PD-1: programmed cell death-1; PD-L1: programmed cell death ligand-1
PACIFIC regimen: CRT followed by IMFINZI12
for stage III patients with PD-L1 ≥ 1%
The PACIFIC study
The PACIFIC trial was a randomized, double-blinded, placebo-controlled, multicenter trial, comparing Imfinzi (durvalumab) vs placebo in patients with Stage III, unresectable NSCLC without progression after CRT.
Study enrolled patients with unresectable, stage III* NSCLC Who had an ECOG performance status of 0 or 112
-

-
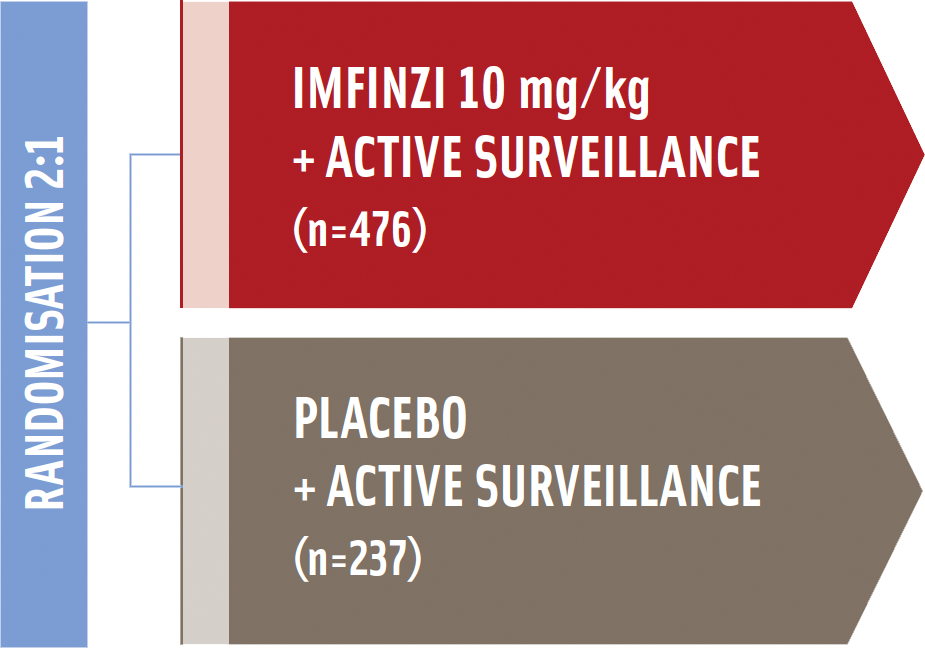
-
Co-primary endpoints§:
- Progression-free survival (PFS)||
- Overall survival (OS)
-
Secondary endpoints§:
- Objective response rate (ORR)
- Duration of response (DoR)
- Health-related quality of life (HRQoL)¶
- Safety and tolerability
V: intravenous, ITT: intent to treat
* According to the Staging Manual in Thoracic Oncology, version 7, of the International Association for the Study of Lung Cancer.
† Patients must have completed their last dose of platinum-based CRT within 1-42 days prior to randomisation in the study.1
‡ Absence of progression following at least 2 cycles of platinum-based CRT.1
§ Measured from day of randomisation and initiation of study drug or placebo.1
|| PFS was based on blinded independent central review using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.1
¶ Patient-reported symptoms, function, and health-related quality of life (HRQoL) were collected using the EORTC QLQ-C30 and its lung cancer module (EORTC QLQ-LC13).1
The PACIFIC study results
-
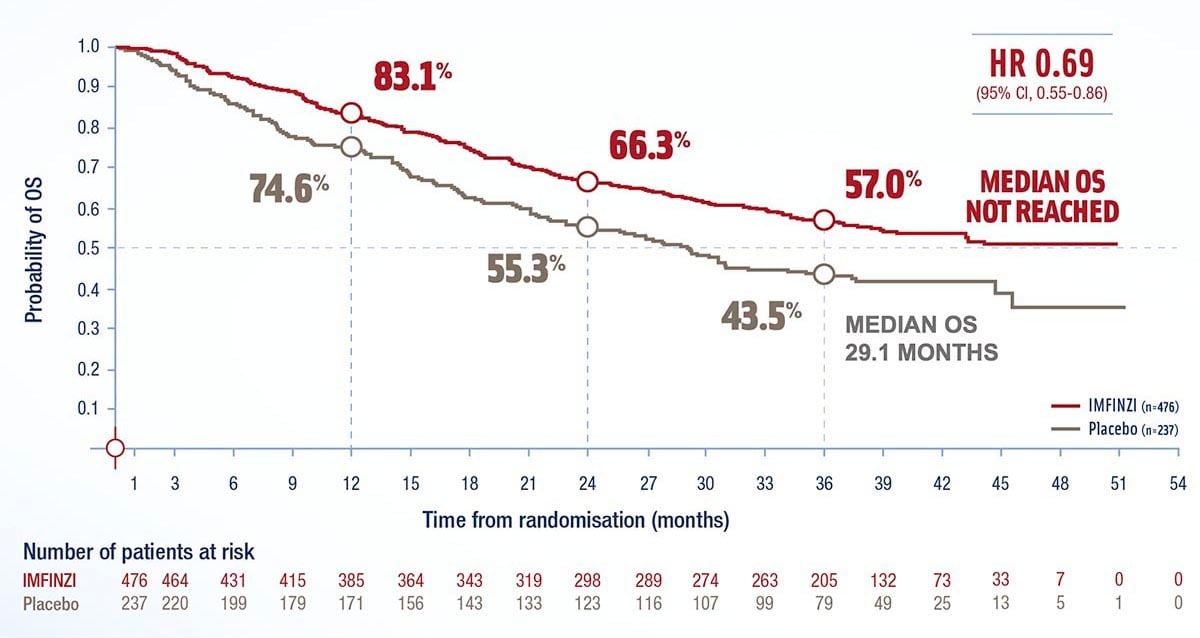
IMFINZI - Improves median OS vs placebo1
in the ITT population*
Overall survival (Intent-to-treat population)1
Post-hoc subgroup analysis according to indication**
Overall survival in patients with PD-L1 ≥ 1%1
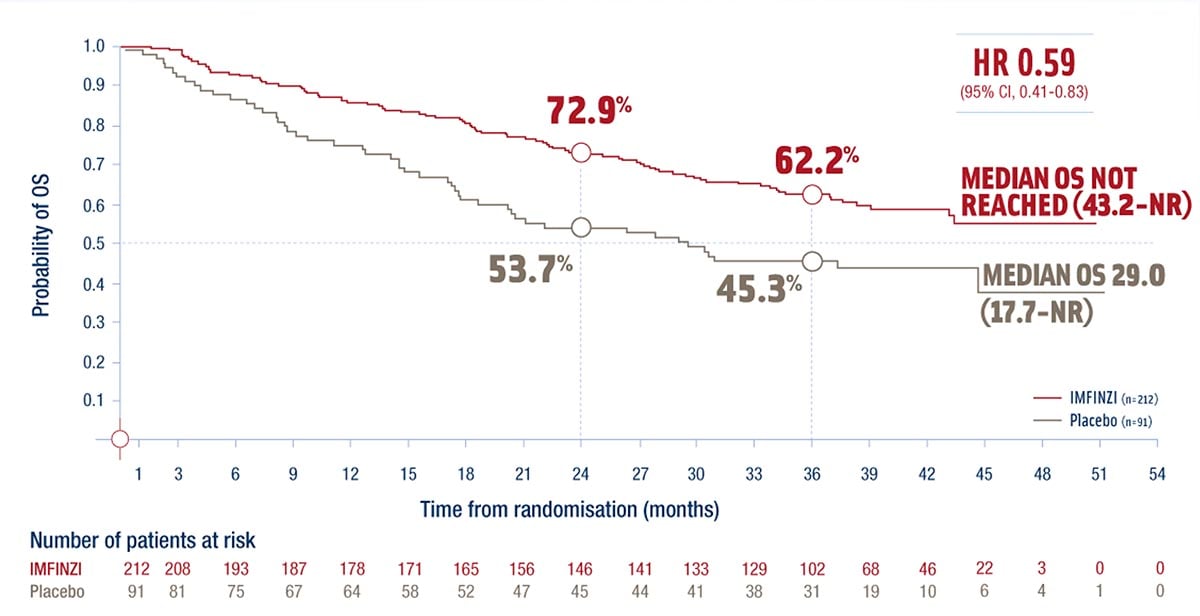
* The results for the PD-L1 ≥ 1% subgroup are based on retrospective, exploratory subgroup analysis requested from the authority. ITT population in the PACIFIC study was enrolled regardless of PD-L1 expression, 63% of patients provided a tissue sample of sufficient quality and quantity to determine PD-L1 expression and 37% were unknown.
**= ITT population in the PACIFIC study was enrolled regardless of PD-L1 expression. The indication is limited to patients with PD-L1 expression ≥1%, based on a retrospective, exploratory subgroup analysis of the ITT population requested from the authority.
ITT: intent to treat; mOS: median overall survival; NR: not reached
-
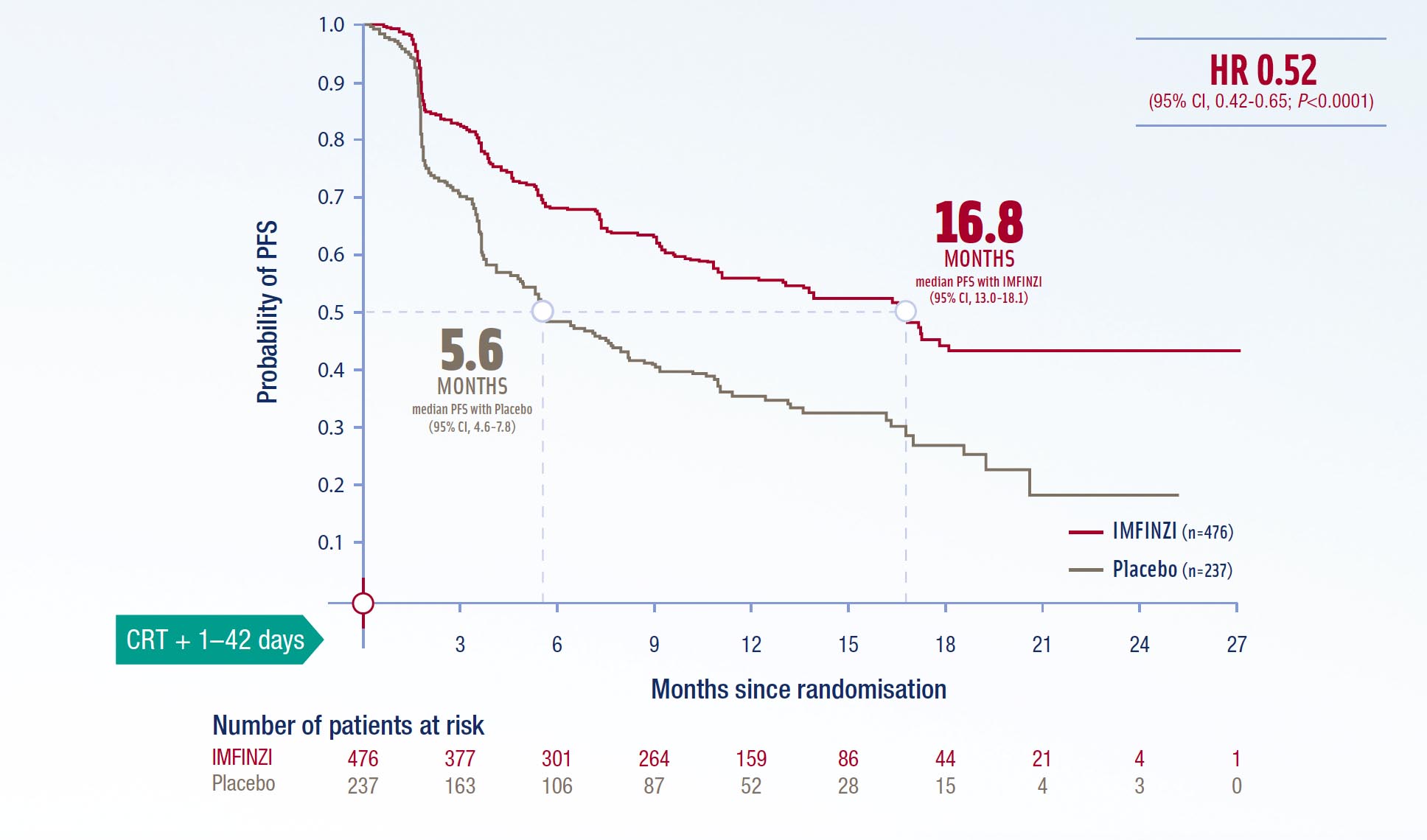
IMFINZI - Improves median PFS vs placebo1
in the ITT population*
Progession-free survival (intent-to-treat population)1
Post-hoc subgroup analysis according to indication**
Progression-free survival in patients with PD-L1 ≥ 1%1
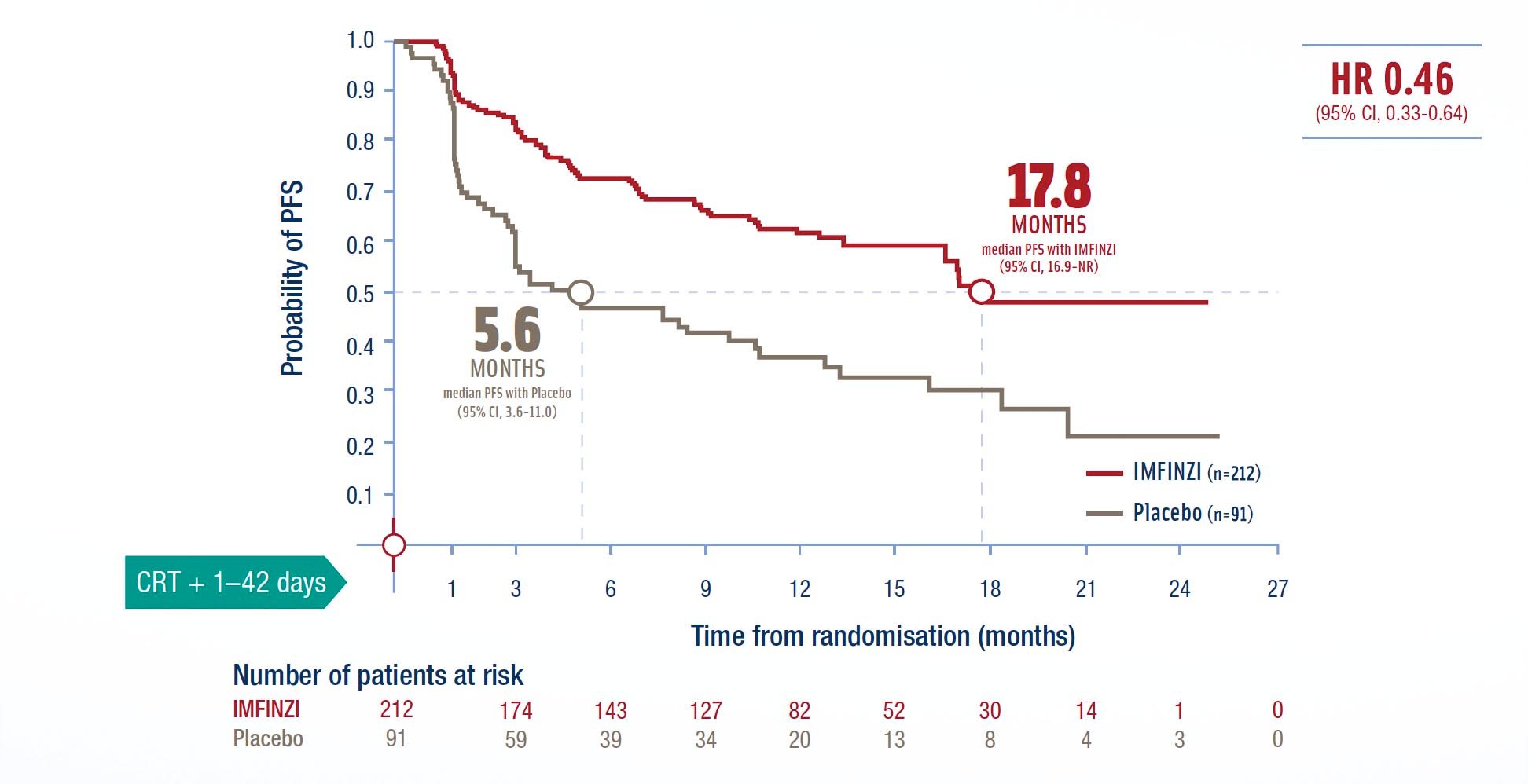
* The results for the PD-L1 ≥1% subgroup are based on retrospective, exploratory subgroup analysis requested from the authority. ITT population in the PACIFIC study was enrolled regardless of PD-L1 expression, 63% of patients provided a tissue sample of suffi cient quality and quantity to determine PD-L1 expression and 37% were unknown.
**= ITT population in the PACIFIC study was enrolled regardless of PD-L1 expression. The indication is limited to patients with PD-L1 expression ≥1%, based on a retrospective, exploratory subgroup analysis of the ITT population requested from the authority.
mPFS: median progression-free survival
Adverse reactions reported with IMFINZI
Adverse reactions reported in ≥10% of patients (very common)
* Fatal pneumonitis and fatal pneumonia were reported at similar rates between the IMFINZI and placebo groups in the PACIFIC study; fatal hepatitis was reported in other clinical trials.3
Immune-mediated adverse reactions reported
with IMFINZI
Any-grade immune-mediated adverse events reported in ≥ 1% of patients12
‡Grade 5 immune-mediated AEs occurred in 4 patients (0.8%) receiving durvalumab and 3 patients (1.3%) receiving placebo.
Prior to prescribing, please read the IMFINZI SmPC for full prescribing information including important safety information on monitoring and management of immune-mediated adverse reactions.
Dosing and administration1
IMFINZI is administered as a 1-hour intravenous infusion1. The dose is weight-based (10mg/kg) as a 1-hour IV infusion once every 2 weeks*.
- IMFINZI is supplied as single-use vials that contain either 500 mg/10 ml or 120 mg/2.4 ml.1 Refer to the IMFINZI SmPC for further information on storage, dilution, and administration instructions.
- Treatment must be initiated and supervised by a physician experienced in the treatment of cancer1
*Administered until disease progression or unacceptable toxicity1
In the PACIFIC study1:
- Treatment with IMFINZI was initiated within 6 weeks of completing platinum-based CRT.
- Infusion-related reactions with IMFINZI were observed in 1.9% of patients vs 0.4% with placebo
Detta läkemedel är föremål för utökad bevakning
Imfinzi ® (durvalumab) 50 mg/ml, koncentrat till infusionsvätska, lösning, L01XC28, Antineoplastiska medel, monoklonala antikroppar, Rx, EF= ingår inte i förmånen. Indikationer: IMFINZI som monoterapi är indicerat för behandling av lokalt avancerad, icke-resektabel icke-småcellig lungcancer (NSCLC) hos vuxna vilkas tumörer uttrycker PD-L1 på ≥ 1 % av tumörceller och vilkas sjukdom inte har progredierat efter platinabaserad radiokemoterapi (CRT). IMFINZI i kombination med etoposid och antingen karboplatin eller cisplatin är indicerat för första linjens behandling av vuxna med avancerad småcellig lungcancer (ES-SCLC). Dosering och administrering: Behandling måste sättas in och övervakas av en läkare med erfarenhet av cancerbehandling. Patienter med lokalt avancerad NSCLC ska utvärderas för behandling baserat på tumöruttryck av PD-L1 som bekräftats med en validerad analysmetod.Varningar och försiktighet: Immunmedierade biverkningar kan uppkomma. För mer information kring hantering av dessa, se produktresumén.
Senaste översyn av produktresumén: 2021-10-19. För ytterligare information och priser se www.fass.se
AstraZeneca AB, AstraZeneca Sverige, 151 85 Södertälje, Tel 08-553 260 00. www.astrazeneca.se
References
- IMFINZI Summary of Product Characteristics; www.fass.se.
- Nationellt vårdprogram Lungcancer (Sverige). Version 2.0. Augusti 2018. Regionalt cancercentrum
- Eberhardt WE, De Ruysscher D, Weder W, et al; Panel members. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26(8): 1573-1588.
- Årsrapport från Nationella lungcancerregistret (NLCR) 2015 (Sverige). Regionalt cancercentrum.
- Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classifi cation. Chest. 2017;151(1):193-203.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379-386.
- Hanna N. Current standards and clinical trials in systemic therapy for stage III lung cancer: what is new? Am Soc Clin Oncol Educ Book. 2015:e442-e447.
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458-5468.
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687-695.
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specifi c effector cells that traffi c to the tumor. J Immunol. 2005;174(12):7516-7523.
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259-1271.
- Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
- Data on File, REF-41250, AstraZeneca Pharmaceuticals LP.
- Data on File, REF-40507.
- Lungcancer i Sverige. 2010 May 11;:1–40

